Our goal is to harness our understanding of protective maternal immune responses into the development a maternal or pediatric HIV vaccine to achieve an HIV-free generation. Some of the research projects in the lab include:
Defining mechanisms of IgG transplacental transfer to the fetus.
Grant Number:
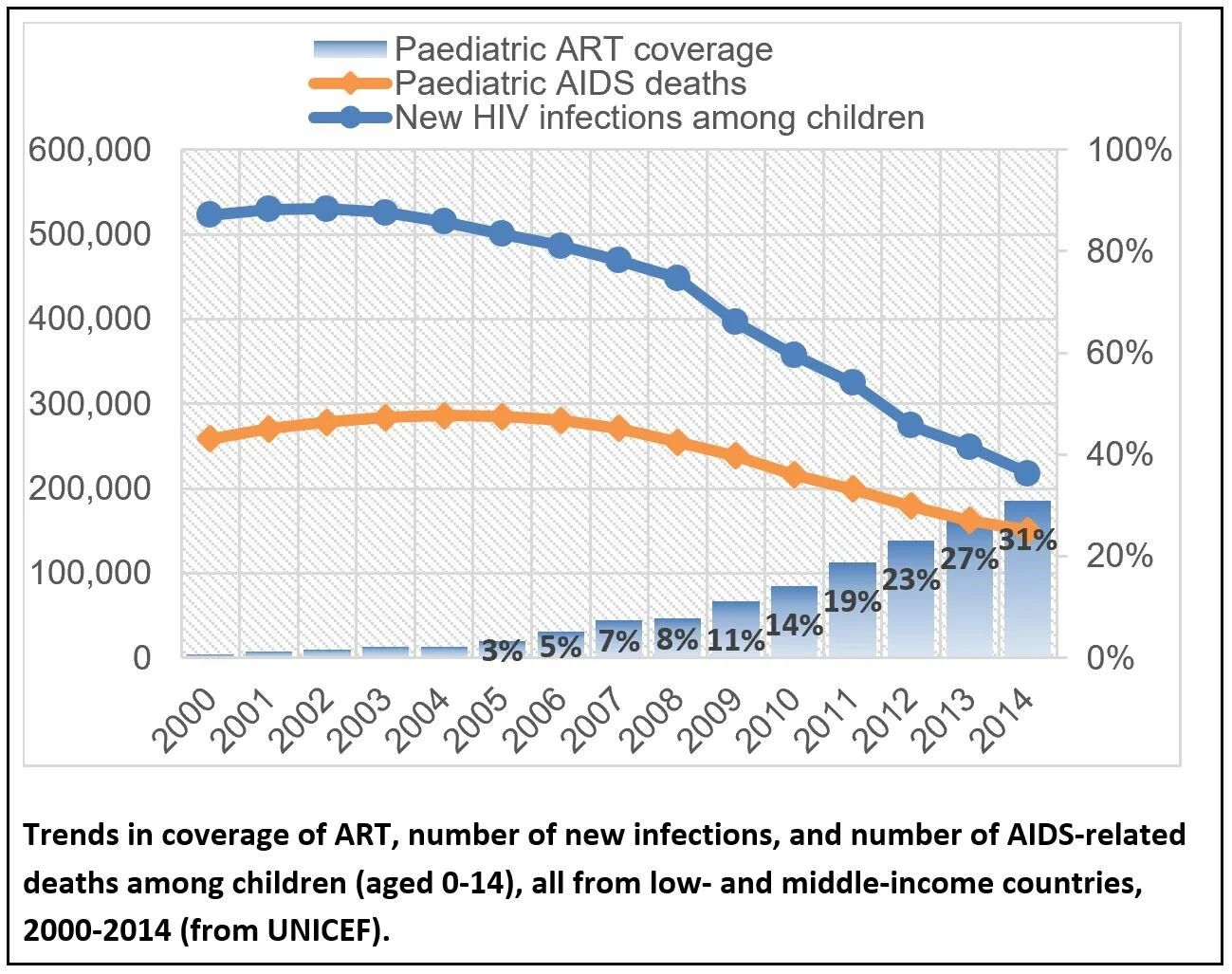
Globally, up to 11% of HIV-infected women still transmit the virus to their infant, resulting in over 400 children each day who acquire HIV-1 (>150,000 infants per year) who will require lifelong HIV-1 therapy(1). In fact, the current pediatric HIV incidence rate is far from reaching the UNAIDS established global goal of <20,000 cases annually by 2020. This rate of vertical transmission is ongoing in spite of 82% of HIV-infected pregnant and lactating women having good access to ART globally
Maternal-infant transmission of HIV-1 is a unique transmission route that occurs in the setting of pre-existing antibodies against autologous viruses, which applies selection pressure to vertically-transmitted virus variants. We have identified maternal antibody responses that predict reduced risk of MTCT, yet determined that viral escape of maternal neutralizing antibodies against mothers’ own circulating viruses is common in infant infections, including escape of plasma bnAb activity. We now seek to define the role of maternal plasma bnAb responses in MTCT and whether viral escape of bnAbs is a risk factor for vertical virus transmission. Understanding the impact of pre-existing bnAb activity on MTCT risk is important to design immunologic strategies that will synergize with ART to eliminate pediatric HIV infections.
Early Life Vaccination to Prevent HIV acquisition during Adolescence
HIVRAD P01 Project Grant #
Nearly 600,000 new HIV infections occur yearly in adolescents/young adults (ages 15-24 years), the only population in which HIV infections continue to rise. The end of the HIV/AIDS epidemic will be achievable only when an effective vaccine regimen can elicit long-term protective immunity in preadolescence, prior to sexual debut. Yet, even the most promising HIV envelope (Env) vaccine platforms have failed to induce highly-protective immunity in adults in preclinical and clinical studies.
The HIVRAD P01 Program Project collaboration will compare immune responses of two of the most promising contemporary HIV vaccination strategies (SOSIP trimer and mRNA) using highly relevant infant and adolescent nonhuman primate models and define strategies that harness unique aspects of infant immune development to produce protective anti-HIV immune responses, such as broad neutralizing antibodies. This collaboration will identify the age group that should be targeted future human clinical vaccine trials in order to provide HIV protection prior to adolescence and provide insight into immune and microbial factors relevant to vaccine design.
Origin, kinetics, and predictors of viral rebound in infants
Grant Number:
Nearly 2 million children are infected with HIV worldwide, and every year more than 150,000 new pediatric HIV infections occur. Postnatal breast milk transmission accounts for at least half of these new infections. Current standard of care commits HIV-infected children to lifelong, daily antiretroviral treatment (ART). A cure is needed to provide HIV-infected children a life without the medical complications, pharmacological burden, and social stigma associated with HIV-1 infection
Interruptions in treatment, perhaps due to poor financial resources, poor access to medical care, or poor adherence, carry a high risk of medical complications due to what is known as “viral rebound”. Using a highly relevant animal model, we seek to define the origin, kinetics, and predictors of viral rebound in postnatally-infected infants, as well as assess the potential impact of immune-based interventions to eradicate pediatric HIV reservoirs.